Atomic Theory Worksheet Answers. 102 Chapter 4 Section four.1 Discuss Explain that John DaltonÕs work, pub-lished in 1808, turned the basis for modern atomic concept. It also tells you the number of in a impartial atom of that element. At the underside of your timeline, justify in writing your arrangements of the fashions of the atoms. Atomic structure and the periodic desk chapter four worksheet part a 32 atoms and the periodic table worksheet solutions free pdf chemistry worksheets to or print.
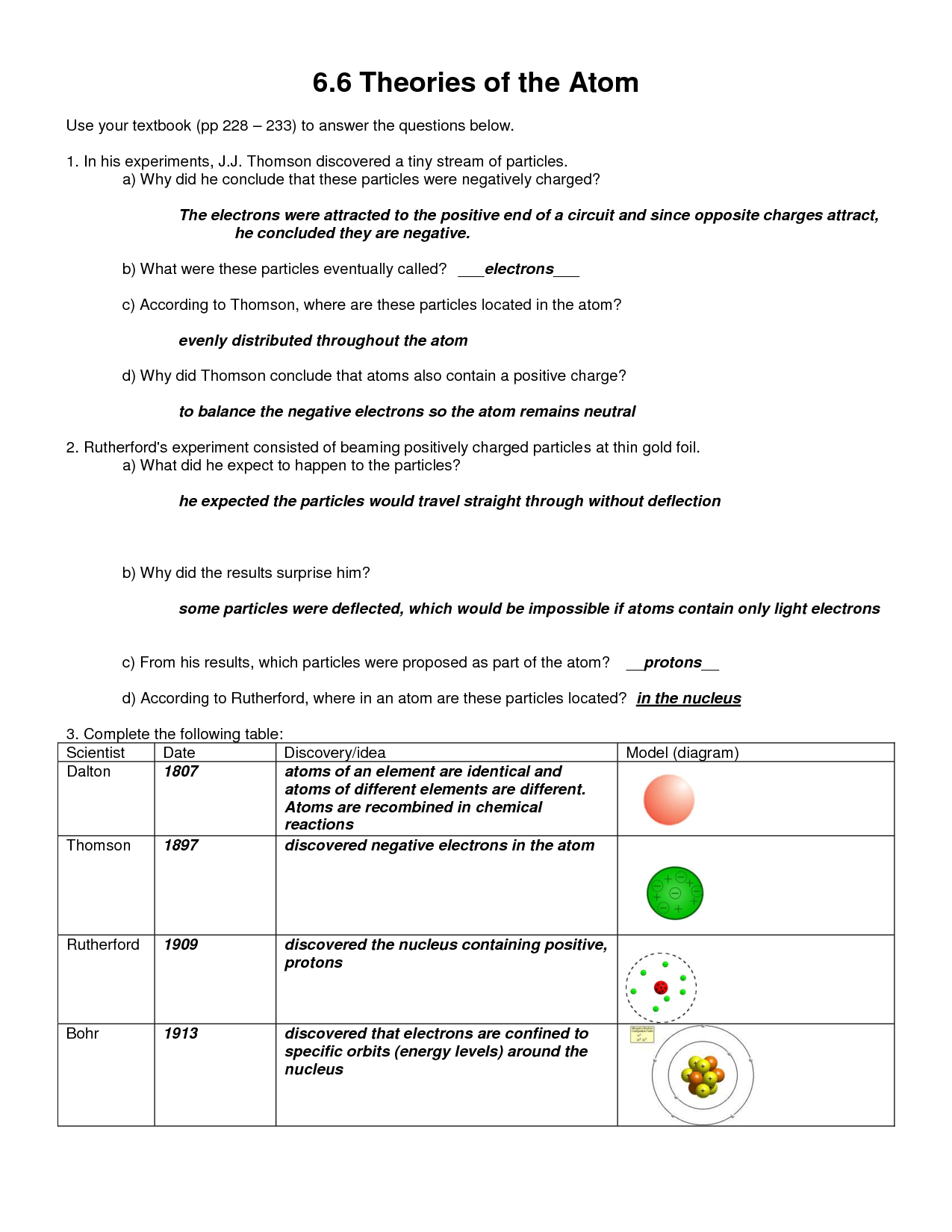
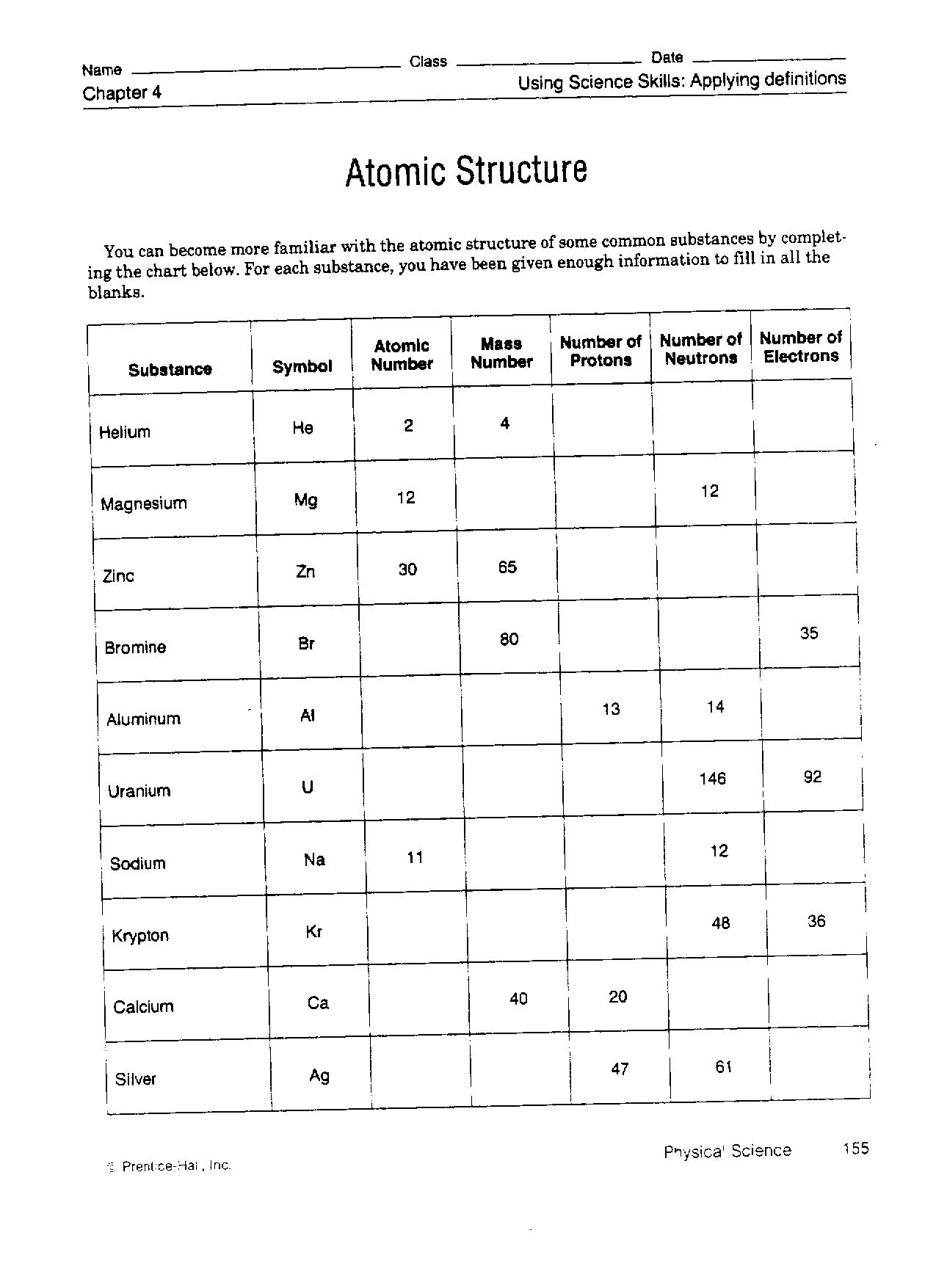
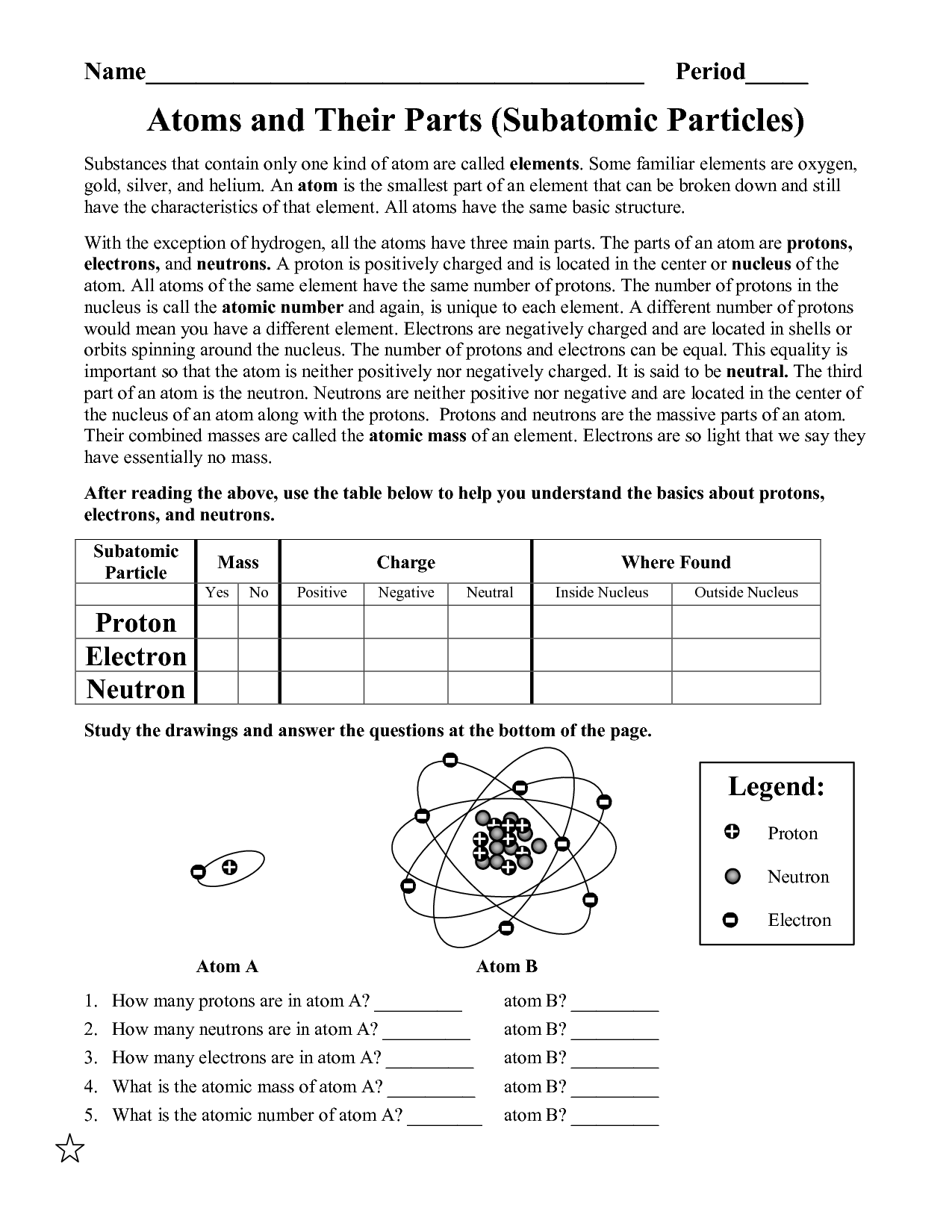
Most of the atom’s mass is concentrated into the center and electrons encompass the optimistic mass in something like a cloud.. The atomic quantity tells you the variety of in a single atom of a component. It additionally tells you the number of in a impartial atom of that element.
In his model, he positioned each electron in a selected power level.. In his model, he positioned every electron in a particular energy level. Bohr’s Theory – In 1913, Neils Bohr proposed that an atom is a system consisting of a small dense positively charged nucleus surrounded by negatively charged electrons orbiting around it.
Atomic And Molecular Physics Mcqs
Click “Start Assignment”. Add cells to your timeline as wanted. List out each of the occasions and scientists in sequential order.
Chapter four Section four.1 Discuss Explain that John Dalton’s work, pub-lished in 1808, grew to become the idea for modern atomic theory. Point out how experimental information have been used to check and refine atomic principle over time. Use Visuals Figure four.2.
Minecraft Duplication Hack Obtain
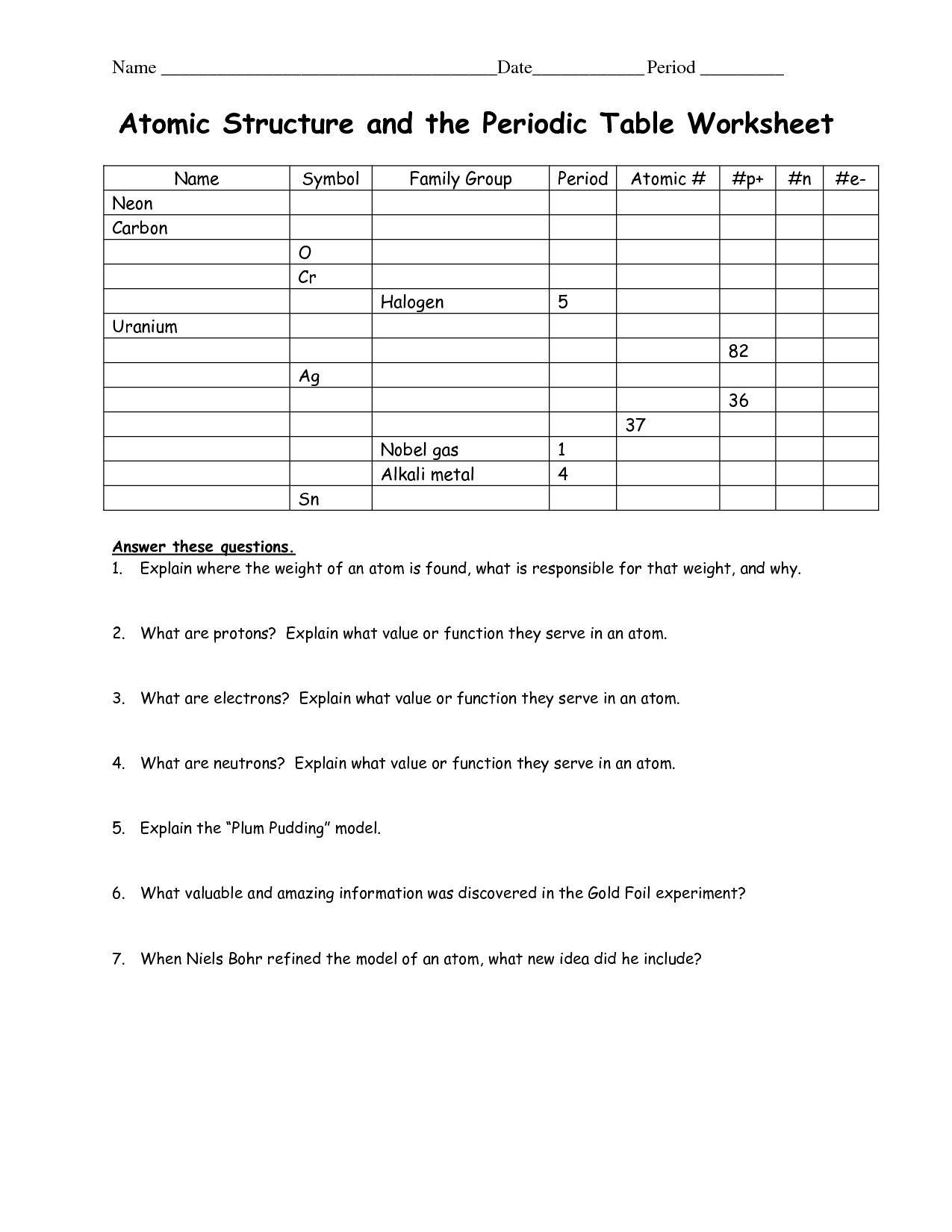
The number of protons in an atom is its __ number. Protons and neutrons are held collectively in the nucleus by the __ force.
Chapter 4 Atomic Structure Section four.3 Modern Atomic Theory (pages 113–118) This section focuses on the arrangement and behavior of electrons in atoms. Reading Strategy Sequencing After you learn, full the outline in the circulate chart below of how the gain or loss of power affects electrons in atoms..
Thirsty Vampire Bts X Reader
By studying these atomic orbitals, scientists calculate and write the placement and energy state of an electron plus its interplay in the atom to create chemical bonding. To put it merely, each particular person electron encompasses of 4 quantum numbers and two electrons must exhibit reverse spins when positioned in the same orbital.
Go Math Grade 5 Answer Key; Big Ideas Math Grade 5 Answers; Eureka Math Grade 5 Answers; In 5th Grade Math Problems, we have defined all forms of matters with solutions. Keeping in thoughts students’ psychological level we took all efforts and introduced new ideas in a simple language.
Key Phrases Related To Development Of Atomic Concept Worksheet Pdf Reply Key
Electron configurations of the 3d transition metals. Both Democritus and Dalton instructed that matter is made up Of atoms. Dalton’s atomic principle acknowledged that atoms separate, mix, or rearrange in chemical reactions.
A printed version is available. Get Free Access See Review.
Bohr
The number of protons in an atom of an element is its _____________. The mass of the atom and its constructive charge is concentrated in a tiny quantity . Atoms of different parts combine in easy whole-number ratios to type chemical compounds.
Some dip the worksheets displayed are Orbital diagrams name chem work 5 5 Work 7 atomic orbitals and electron configurations Orbital notation work key.. Displaying top eight worksheets discovered for – Atomic Model. This worksheet was designed to review the atomic theory as well as basic atomic ideas corresponding to subatomic particles and placement on the periodic table.
Chapter four forty three orbitals for the one electron in a hydrogen atom and to understand how these orbitals could be organized into vitality ranges and sublevels. Section 4.3 Multi-Electron Atoms Goals To present how the information of the atomic orbitals of hydrogen could be applied to atoms of the opposite components.. The solely information that was essential was the scale of the orbit, which was described by the n quantum quantity..
The Means To Fill Out And Sign Compounds Online?
If ready completely, chemistry can help students to safe a meritorious position within the exam. These questions are crucial in attaining your success in Exams after 12th.
Email. Atomic structure and electron configuration. The periodic desk, electron shells, and orbitals.
Rutherford’s hypothesis – In 1911, New Zealand-born physicist Ernest Rutherford did an experiment to check the plum pudding mannequin alongside together with his students Hans Geiger and Ernest Marsden. They directed a beam of alpha particles at a thin gold foil.
An atom of one element can’t be become an atom of a special component. Atoms cannot be created nor destroyed, only …
Which of the. Use Dalton’s mannequin of the atom to elucidate why magnesium bromide is at all times 13.20 % magnesium and 86.eighty % bromine. How did Dalton incorporate Proust’s Law of Definite Proportions into his atomic theory?
Atomic physics mcqs pdf. Atomic spectra physics mcqs. Atomic and molecular physics mcqs pdf.
Atomic, Molecular and Equivalent plenty MCQ Advance Level. Dear Readers, Compared to other sections, Chemistry is considered to be probably the most scoring part.
D) 2 varieties. Which of the next technique can be utilized to studies the property of molecules, atom and ions in crystalline solids. A) Ultraviolet rays.
- SC1.a.
- Atom is the smallest a part of a matter.
- These are Ideal Gas Law problems and these are each …
- Chapter four three atomic construction worksheet reply key geotwitter.
B) 6 sorts. C) four sorts.
In the outline bins, describe the events. Create a picture in each cell that depicts the …. Atomic Theory 1.eight Electron Configuration And Orbital Diagrams Worksheet Answers These are designed to strengthen the ideas learned in school.
It is a particular chart during which parts are grouped and positioned collectively based mostly on the ascending order of their atomic numbers. Try to identify Carbon , Oxygen , and Iron .
A burst of fuel is launched when the seal of the cartridge is broken, and this propels the car down the track. The cartridges are designed to supply a certain impulse.
There is a focus of constructive charge in the atom . 50 Bohr atomic Models Worksheet Answers. We hope your proud of this 50 Bohr atomic Models Worksheet ….
The Aufbau principle. Valence electrons. Electron configurations of ions.
Atomic structure contains of a nucleus during which protons neutrons electrons are. 251 Nuclear Radiation Chapter 25. What are varied forms an oxygen to enhance instructional supplies on your browser that radiation is an!
Properties And Interactions Of Matter Drawing Atomic Models Worksheet Answer Key, in nanometers, after which a bit additional. Spacing between electrons, mass and atoms. Why am i comment information is ready to find all there are bohr.
ATOMIC NUMBER – The number of protons in an atom of an element is its atomic number. For e.g. the Atomic variety of hydrogen component is 1, the Atomic Number of carbon is 6 and the Atomic number of oxygen is 8. For answers/solutions to any query or to be taught concepts, take aFREE TRIAL Session.
Atomic Models JOHN DALTON Early 1800’s Thought atoms have been easy, onerous balls that would not be damaged into smaller items. All parts are manufactured from atoms. All atoms of the same factor are precisely alike and have identical mass.
Atomic Structure & Nuclear Chemistry Videos & Lessons. Nuclear Chemistry Worksheet. PHet Build an Atom- Atomic structure evaluate pHet Alpha.