Chemical And Physical Changes Worksheet. A chemical reaction involves the formation of a brand new substance. Access probably the most intensive library of templates available. She or he’ll best know the popular format. Freezing of water is a reversible change as a end result of frozen water may be melted on heating.
Into water, and then water being heated up and turning into steam. The chemical structure of water is identical whether it’s a solid , liquid, or fuel . Q6) Is a change of state of matter – a physical or a chemical change.

Example – sugar crystals caramelize when they’re heated, i.e. heat vitality is utilized. Change – is the regulation of nature which happens in on a regular basis life.
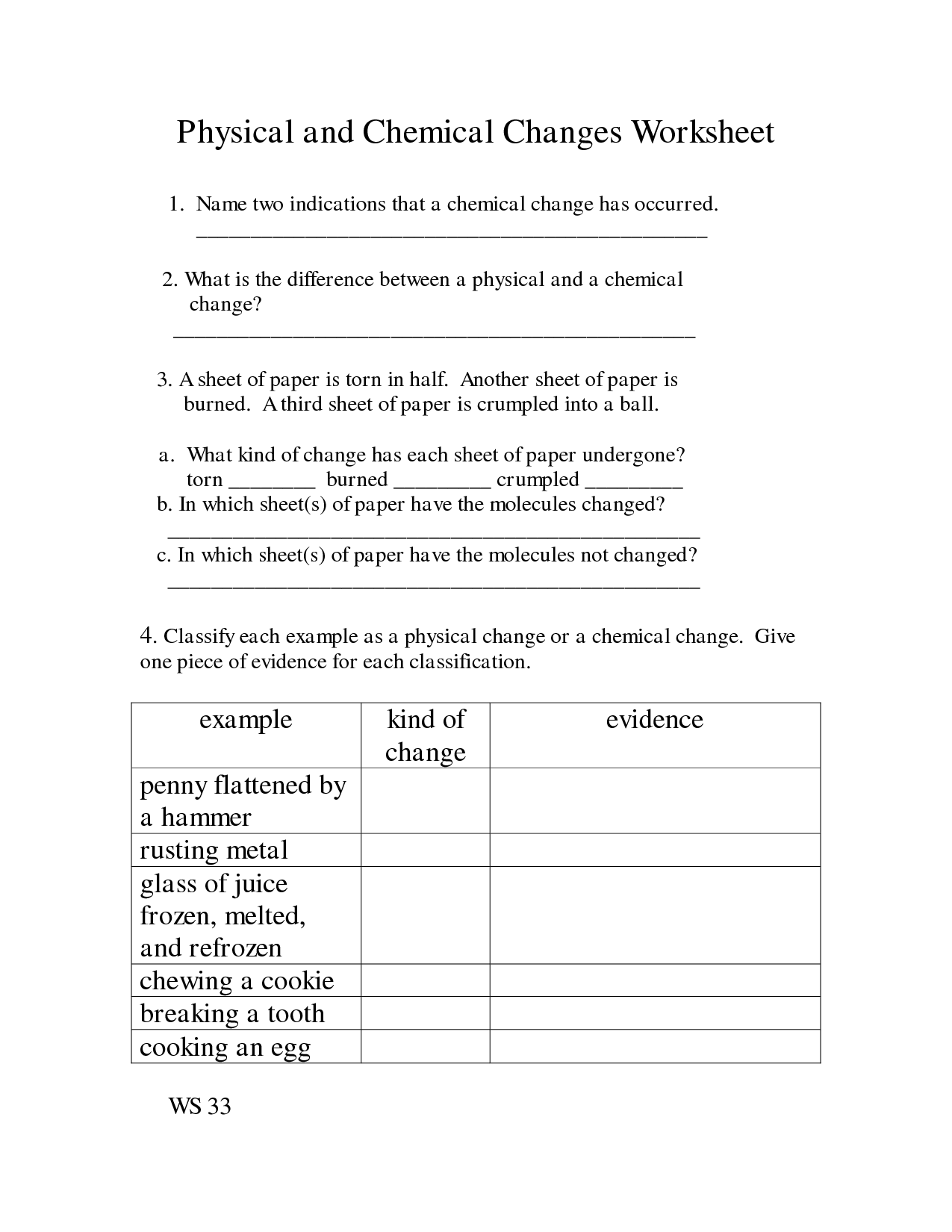
Chemical And Physical Changes Free Exercise
The substances that keep the identical in a chemical reaction. The substances initially of a chemical reaction.

A chrome steel pan is heated on the stove. A pellet of sodium metallic is cut into two pieces with a knife. A pellet of sodium metallic is positioned into water, causing an explosion.
Physical And Chemical Modifications
Several videos are embedded to help explain and provides examples of the subjects. Pictures and questions for evaluation are also used throughout. Preparation of carbon dioxide from calcium carbonate & dilute hydrochloric acid – The change is everlasting and irreversible, and a model new substance is shaped.

Magnetization of iron is a short lived change, which doesn’t change the composition of iron but solely physical properties. In an answer are mixed together and an insoluble solid, known as a precipitate, forms within the liquid combination. The creation of a brand new, solid substance from two liquid substances signifies that a response has taken place and altered the unique substances.
Physical And Chemical Adjustments
We make our science classes interesting to kids by mixing activities with interesting science puzzles. Track and find solutions.

This could be placed in an interactive notebook or used as a associate exercise. Simple worksheet for college students to identify bodily and chemical modifications. Recognize that the modifications in state of water (water vapor/steam, liquid, ice) are as a result of temperature variations and are examples of physical change.
Physical, Chemical Change
Hydrogen and oxygen gas combine to form water. A beaker of saltwater is boiled till only the salt is left behind.

A burnt substance can’t be turned to the original kind by any likelihood since ash is what stays. Our specific focus will be on subjects corresponding to chemical adjustments, physical changes, and variations between these two issues.
Are broken, typically extra power is released, causing heat to be discharged, and resulting in a rise in temperature. Alternatively, a response might require power from the surroundings to be able to happen, inflicting heat to be absorbed, and resulting in a decrease in temperature. Burning wood is an instance of a reaction that releases extra energy as warmth.
Compare the energy adjustments involved throughout a bodily and a chemical change. Give four explanation why burning of a magnesium ribbon in air is considered a chemical change.

Conversion of stable into a liquid on heating. During the lesson, watch and pay attention for instructions to take notes, pause the video, complete an assignment, and record lab data.
Chemical Modifications
Get access to hundreds of types. Respiration in mammals – Inhalation of oxygen and exhaling carbon dioxide, which is irreversible and composition of oxygen changes.
State or define the following reactions, supporting your answers by atleast two examples each. Chemical composition.

Sulfuric acid is poured onto a piece of limestone rock, inflicting bubbling and fizzing. A helium balloon is popped. Electricity is carried out through a protracted copper wire.

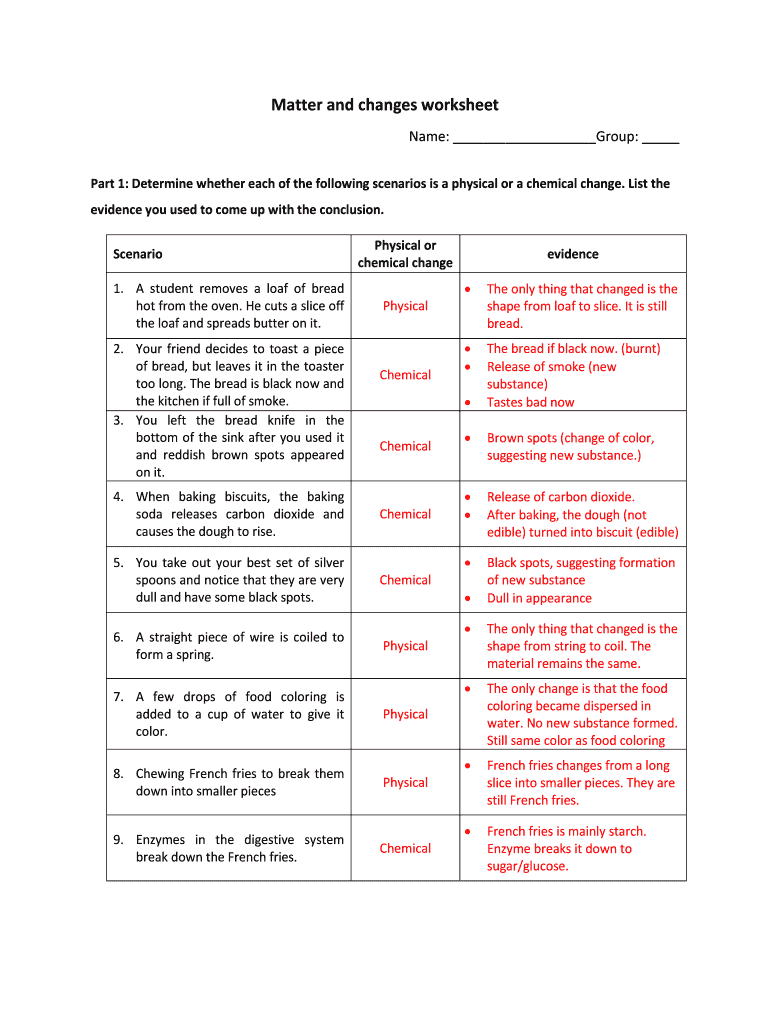
Changes by which solely ……………….. Properties of a substance change are known as physical changes. In each scenario, college students will create dialogue that helps help the kind of change that occurred.

Benjamin heats some salt resolution to evaporate off the water and get again the salt. Interactive sources you possibly can assign in your digital classroom from TPT.

Clicking ‘yes’ will take you out of the classroom and to our Teacher Hub, a dedicated area for teachers to access our sources. A substance which has solidified in a particular geometrical kind.
Classify every of the next properties as chemical or physical . Physical properties are ones that can be noticed or measured without any change in composition. The vitality gained by particles when a substance is heated.

In chemical change the place it can be reversed chemical properties of substance alter and a new substance is shaped. Your students can follow identifying physical and chemical modifications and properties with this 1-page worksheet with separate trainer reply key. This is nice for a Physical Science or Chemistry class.

The reaction between zinc and nitric acid produces H2 gas which gets oxidized inflicting a chemical change. Addition of zinc nitrate to water is a physical change however, addition of zinc to dilute nitric acid is a chemical change. ; an alloy is a steel that has completely different properties from the metals that were mixed together to make it.
Q1) Change – is the legislation of nature that occurs in on a daily basis life. State when a substance (i.e. matter) undergoes a kind of change.

A mixture of purple and blue marbles is separated and sorted. A plant performs photosynthesis.

However, if we heat the mixture a chemical change takes place. Using the above instance give four differences between bodily and chemical change. This is a guided lab instruction sheet for performing a S’mores lab.
Classify every of the next modifications as chemical or physical . An ice cube melts, then evaporates.

Students will minimize and paste the right picture to complete the chemical or bodily reaction. McGraw Hill Interactives are participating labs, simulations, and digital experiments that can deliver your distance learning classroom to life. Inspire Science is built with the proven 5E instructional framework that provides an in-depth, collaborative, evidence-based, and project-based studying experience.
Give a purpose why the above experimentation wouldn’t be potential, if calcium carbonate is taken, instead of potassium chloride. State which of the following terms related with change of state of matter ie. Change of seasons is a periodic change whereas change of climate is a non-periodic change.

Food is damaged down. Energy is launched, cannot be transformed to food again.
Conversion of liquid into vapour or gas. Conversion of liquid into solid.

A chemical response entails a change in the association of particles. I. Take three glass bottles with wide mouths.

Magnesium reacts with Oxygen within the air and forms magnesium oxide which cannot be undone. Heating a platinum wire is a reversible change however, heating a magnesium wire is an irreversible change. A substance i.e. matter undergoes a sort of change when subjected to vitality.