Structure Of The Atom Worksheet. State the traits of nucleus of an atom. Our expert science tutors break down the topics through interactive one-to-one periods. There are many properties of atoms to know and research. Free Sample Papers with solutions for Class 9 Science, obtain…
Describe the mannequin of atom given by J.J.Thomson. Describe the particle scattering experiment performed by Rutherford with a diagram. ______ spin around in the empty area across the nucleus.

Two or extra atoms held together by covalent bonds. There is a positively charged centre in an atom called the nucleus. He additionally proposed that the size of the nucleus may be very small as compared to the dimensions of the atom and almost all of the mass of an atom is centred in the nuclei.
Frequently Requested Questions
Download all VBQ for Class 9 Science in pdf free. Download HOTs Questions for Class 9 Science for all essential matters in Class 9 Science primarily based on CBSE NCERT syllabus and latest sample.
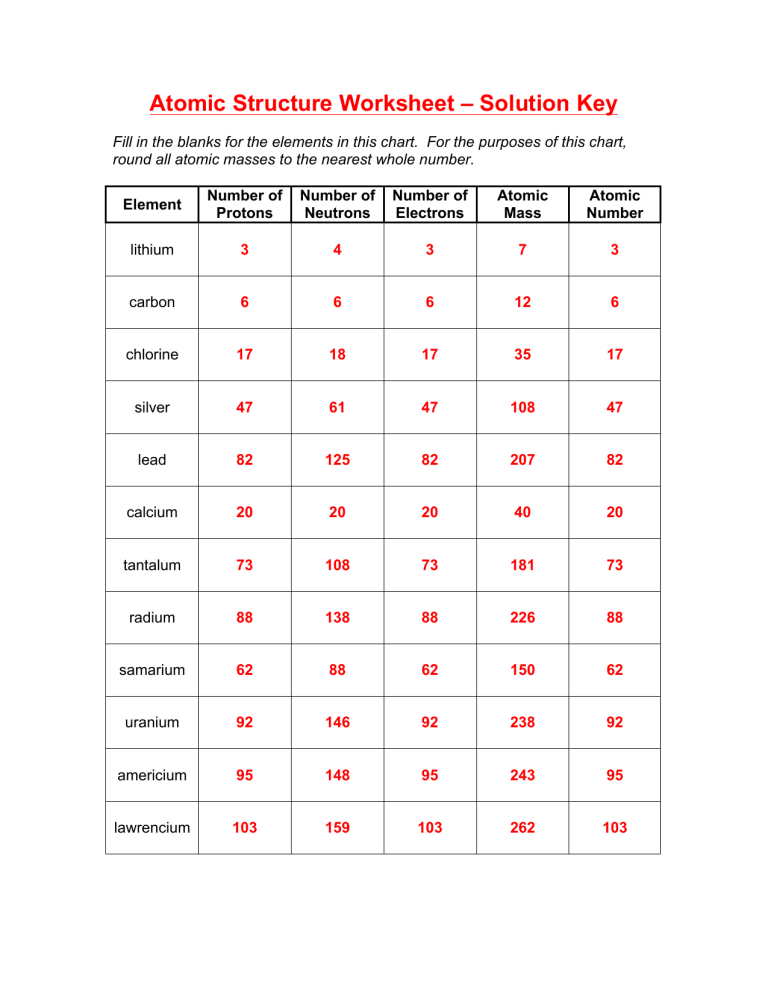
And finally, the charge of an atom is set by the variety of protons and electrons within the atom. Electrons within the Bohr model follow the number of parts within the rows of the periodic desk.
Extra Science Interactive Worksheets
Then colour the correct number of electrons for every orbit. Remember, fill the orbit closest to the nucleus first, however never exceed the quantity every orbit can maintain.
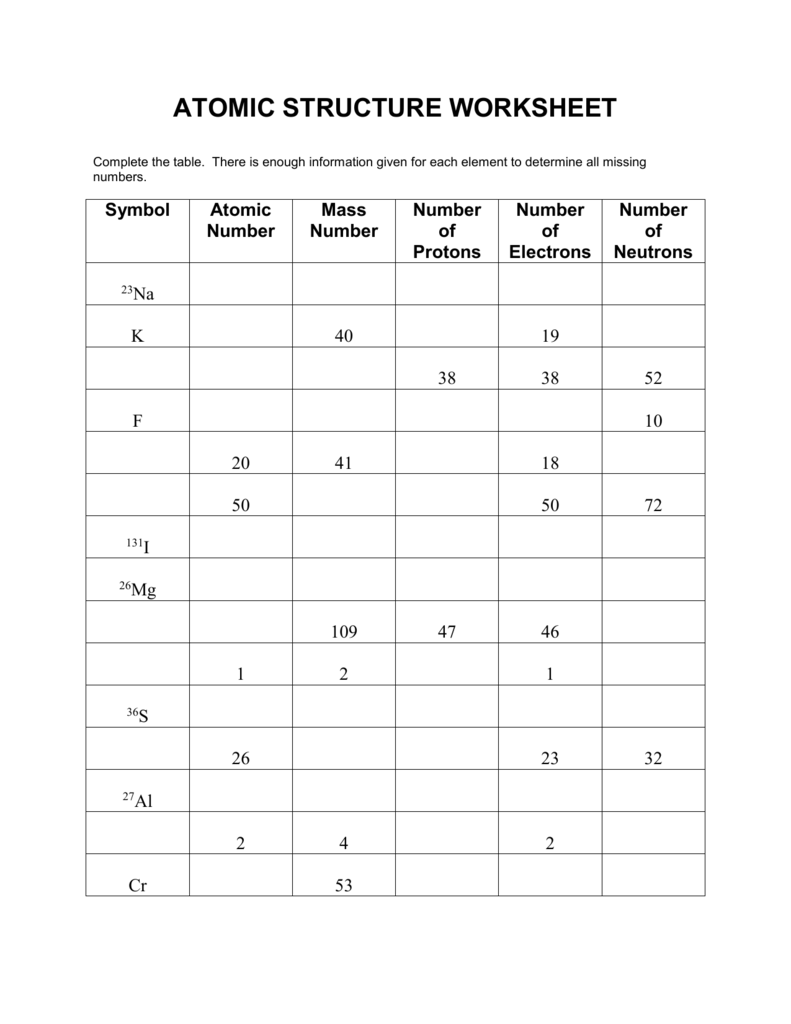
Drawing Bohr Models 1. Write the number of neutrons and the variety of protons within the nucleus. Draw the first power level.
More Atoms Interactive Worksheets
Elaborate the postulates put ahead by E. Rutherford about the structure of atom based mostly on the a -particle scattering experiment. If K and L shells of an atom are full, then what could be the whole number of electrons within the atom?

What kind of charge does a neutron have? Bohr Model Drawing Draw a Bohr mannequin of an . Atom in the space under.
How Do You Discover The Variety Of Electrons?
Displaying all worksheets related to – Structure Of The Atom. Access latest VBQ, Value Based Questions for Class 9 Science as per CBSE and NCERT syllabus.

What are valence electrons? Give example to explain, the method it helps in finding the valency of an atom. An atom is taken into account steady if the outermost shell is crammed with electrons.
Cbse Science Challenge 2021 22
The species A and B are isotopes, as they have identical atomic number however completely different mass number. For answers/solutions to any query or to be taught concepts, take aFREE TRIAL Session.
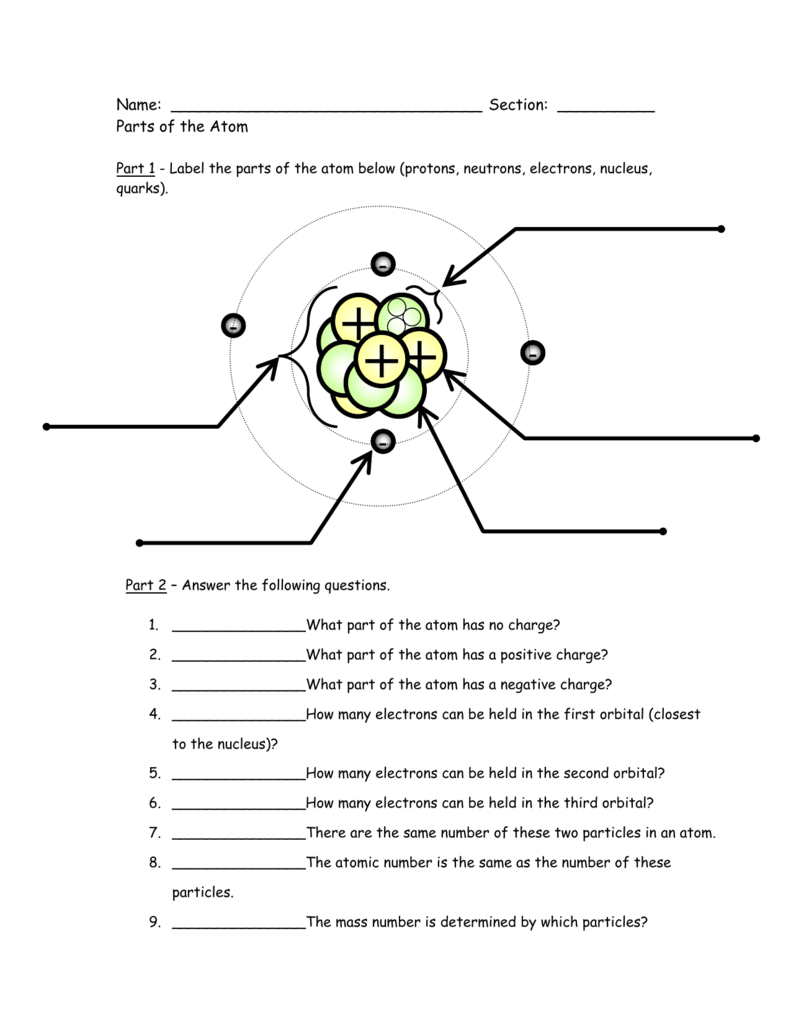
5.The atomic number of chlorine is 17 and mass quantity is 35. The relative number and sorts of atoms in a given compound are fixed.
Bohr Model of the hydrogen atom attempts to plug in sure gaps as advised by Rutherford’s mannequin. At the very middle of an atom is the nucleus, which is made up of small particles known as protons and neutrons. Protons are very small, positively-charged particles, and neutrons are neutral particles that don’t have any cost.
State the traits of nucleus of an atom. 2.Mention any two drawbacks of Rutherford’s mannequin. Iii)Whole mass of an atom is concentrated in its centre.
The protons and neutrons are at the middle of the atom, the nucleus, with empty space around it. The electrons whirl across the nucleus within the empty space, completing billions of journeys in less than a millionth of a second. The paths of these electrons are completely random.

C) Nucleus of an atom is heavy and positively charged. CBSE Class 9 Science Worksheet – Structure of Atom.
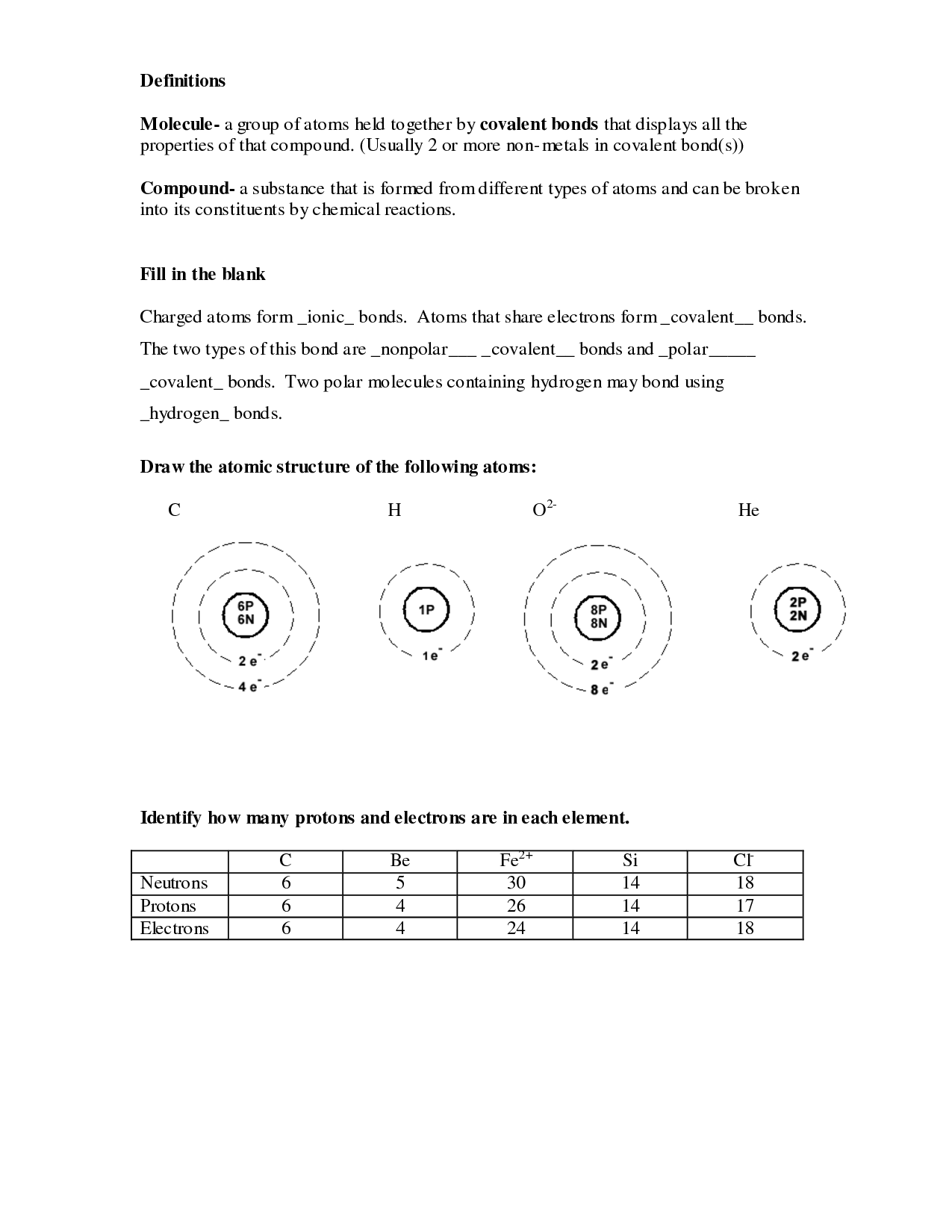
Some isotopes type naturally by way of changes within the nucleus of the atom. The actual variety of protons and neutrons in an atom could endure a change. When this happens to the nucleus, the atom turns into an atom of a different element.

Hence, they’ve completely different mass number. Name the three subatomic particles of an atom. Atoms can’t be created, destroyed or transformed into atoms of other parts.

Free Sample Papers with options for Class 9 Science, obtain… An component 12X24 loses two electrons to form a cation which combines with the anion of element 17Y35 fashioned by gaining an electron. Almost the entire mass of the atom is concentrated within the nucleus.

NCERT Exemplar Problems for Class 9 Science for all topics, Download Exemplar Solutions for Class 9 Science and download in pdf free. NCERT Exemplar Solutions… Click here to obtain NCERT Solutions for questions of Class 9 Science NCERT Book.
Displaying all worksheets associated to – Structure For The Atom.
Draw the electrons in the vitality ranges according to the rules below. Make positive you draw the electrons in pairs. Keep observe of how many electrons are put in every degree and the variety of …
Atomic construction – igcse. How do you need to examine today?

Download Worksheets for Class 9 Science made for all important topics and is out there free of charge obtain in pdf, chapter wise assignments or booklet with… In terms of half time. The time taken by half of the atoms of radioactive factor to disintegrate known as its half-time.

Compare these properties with the properties of electron. It signifies that the constructive charge of the atom occupies a very little area. All isotopes of a component consist of different number of neutrons in their nuclei.

Brainscape Find Flashcards Why It Works Educators Teachers & professors Content … Atomic Structures, Solubility, Priceples of chemistry 1b Show Class chemistry igcse.
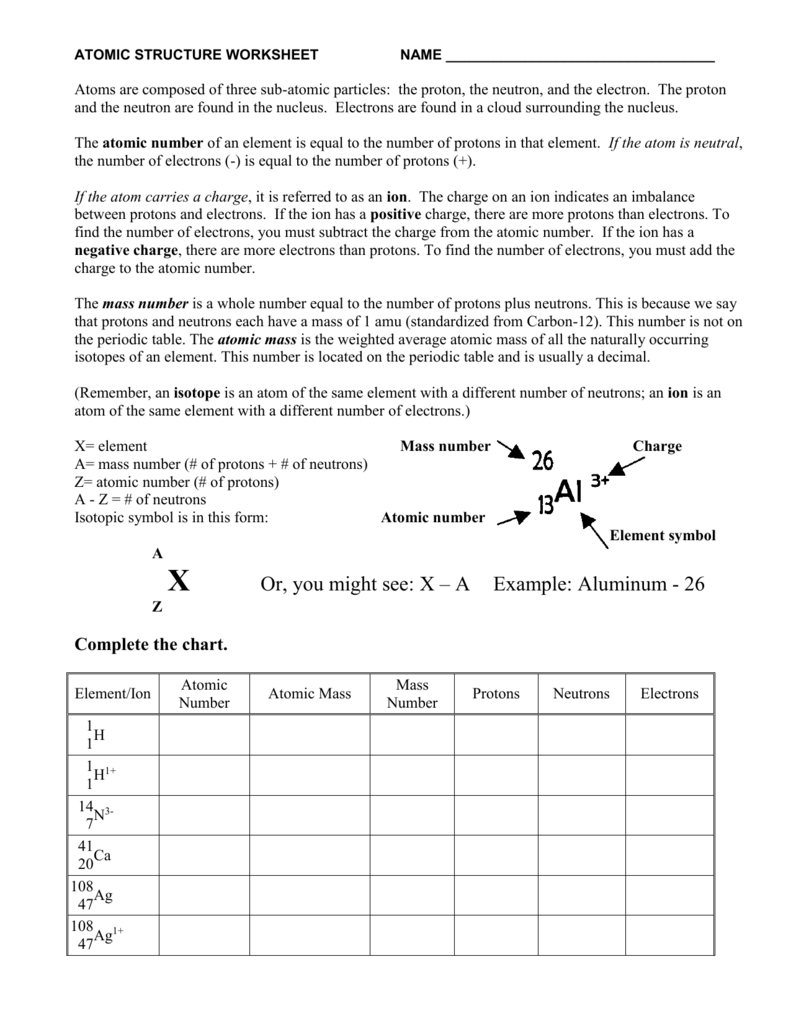
Name a component which has one electron, one proton and no neutron. Isotopes of an element have identical digital configuration.
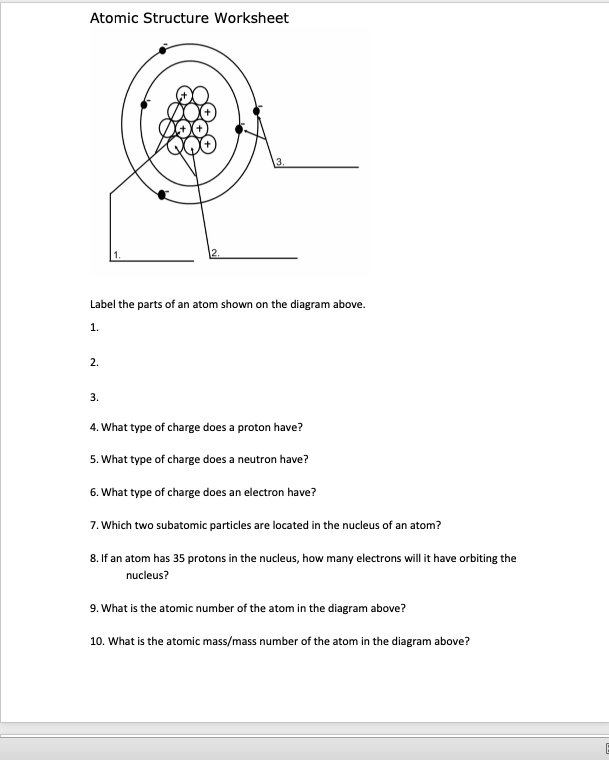
There are many properties of atoms to know and examine. The tiniest bit of something is the atom. The tiniest little bit of gold is one atom of gold.
Thomson proposed the mannequin of an atom to be much like a christmas pudding. The electrons are studded like currants in a positively charged sphere like Christmas pudding and the mass of the atom wassupposed to be uniformly distributed.

Focus your learning with a path. Take a follow test. Get sooner at matching phrases.

Our mission is to supply prime quality online tutoring companies, using cutting-edge Internet expertise, to excessive school students worldwide. Download syllabus for Class 9 Science issued by CBSE and NCERT for 2021. Download newest curriculum with necessary subjects, chapter weightage, subject sensible marks…
This adjustments the mass variety of that atom, creating isotopes of that atom. Most parts have more than one isotope.

Download NCERT books for Class 9 Science, complete book or every chapter in Science e-book for Class 9 in pdf. Also obtain assortment of CBSE books for Class 9… Download latest 2021 Sample Papers for Class 9 Science as per CBSE NCERT sample and syllabus.

Access NCERT Solutions for Class 9 Science. Download options for Science…